Pipeline
One critical path: Inflammation
Four targets
Five potential treatments
At Yaqrit we are developing treatments for patients at each stage of advanced liver disease, built around a deep and new understanding of the role of inflammation and the body’s response to liver injury in the progression of the disease.


Acute and chronic therapies to treat and prevent advanced liver disease
1 L-ornithine phenylacetate 2 rare genetic disease in children
HE: Hepatic encephalopathy; UCD: Urea cycle disorders; ACLF: Acute-on-chronic liver failure; PSC: Primary sclerosing cholangitis; IBS: Irritable bowel syndrome
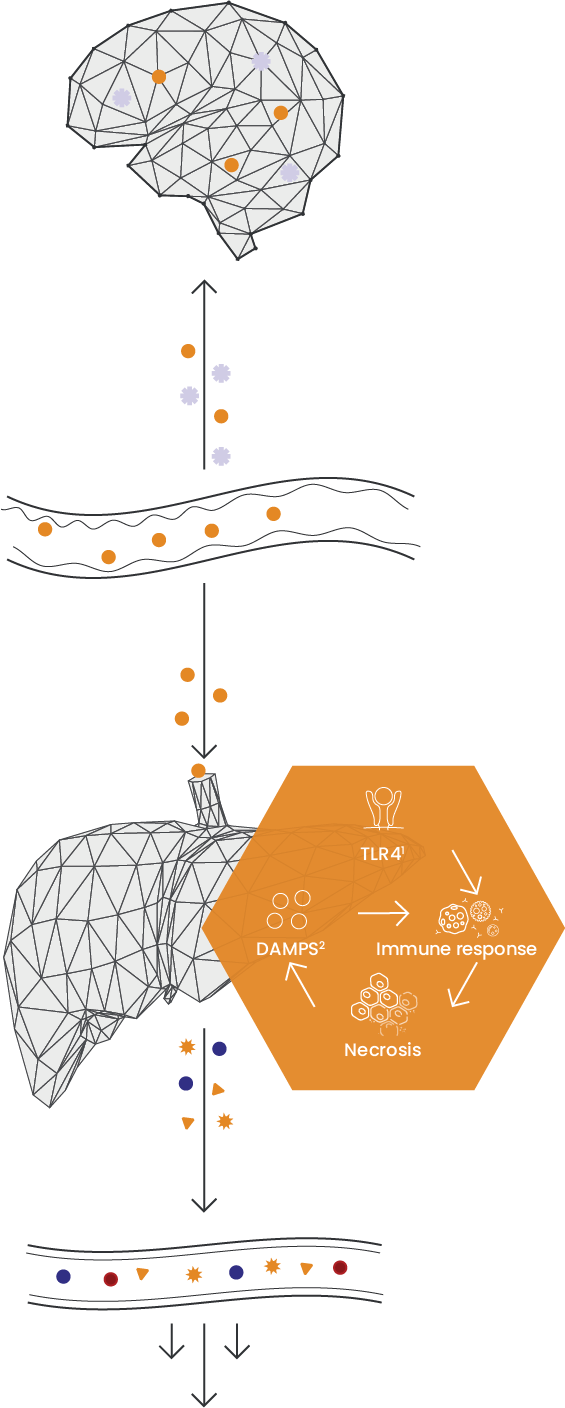
A build-up of ammonia in the blood leads to neurological dysfunction, coma and death
Dysbiosis and leaky gut
Inflammation leads to a cycle of liver cell death
Loss of liver function leads to further aggravation of liver and other organ(s) failing
Organ Failure
Ammonia scavenger
YAQ006 & 007
Prevent and treat recurrence of hepatic encephalopathy
Removes ammonia in the blood and brain reducing neuroinflammation and neuronal cell death, reversing neurological dysfunction.
Advanced microbiome therapeutic
YAQ001
Prevent and treat decompensated cirrhosis
Disrupts the gut biofilm restoring the microbiome thereby reducing gut and systemic inflammation and gut permeability.
TLR4 antagonist
YAQ005
Prevent and treat ACLF grade 1 and 2;
Reduces sensitivity to endotoxin, modulating inflammation and mitochondrial function.
Extracorporeal liver support
YAQ002
Treat ACLF grades 2 and 3;
Removes endotoxin in the blood and replaces damaged albumin, modulating inflammation.
Organ Failure
Organ Failure
Organ Failure
Organ Failure
References
Toll-like receptor 4, a protein in humans that is encoded by the TLR4 gene.
Damage Associated Molecular Patterns.
Macnaughtan J. (2014). Modulation of the gut-liver axis in cirrhosis with activated carbon. Available at: https://discovery.ucl.ac.uk/id/eprint/1547585/1/PhDThesisMacnaughtanFINAL040417.pdf
ADVANCED MICROBIOME THERAPEUTIC
- Designed to remove harmful bacterial toxins from the gut before they leak to the liver and other organs
- Disrupts the gut biofilm preventing harmful toxins leaking from the gut into the body and restoring the microbiome Oral therapeutic with unique engineered physical structure adsorbing both large and small molecules
- Potential oral treatment for previously hospitalised cirrhosis patients who have recovered and are being discharged

TLR4 antagonist
- Targets the TLR4 pathway’s inflammatory cascades and immune responses that drive cell death
- Being developed with Takeda Pharmaceuticals for ACLF patients through a joint venture, Akaza Bioscience
- Yaqrit has plans to develop TLR4 antagonists for other indications such as prevention of ACLF, treatment of hyperammonaemia and urea cycle enzyme disorders
Dialive
- Intensive care treatment for patients with ACLF grades 2-3
- Dual filtration removes bacterial toxins, and replaces dysfunctional albumin
- Targets cytokine storm

Clinical Development Pipeline
Advancing a clinical stage pipeline designed to address each stage of liver disease from diagnosis of decompensated cirrhosis in a hospital setting, to critically ill patients in intensive care.
Target 1
Removes endotoxin, reduces bacterial translocation and improves organ function
Phase
Phase 21
Decompensated cirrhosis
NASH 2
PSC 3
Target 2
Reduces sensitivity to endotoxin, modulating inflammation
Phase
Phase 2
Treatment of ACLF
Prevention of ACLF
Hyperammonaemia
Urea cycle enzyme disorders
Target 3
Removes endotoxin in the blood and replaces damaged albumin modulating inflammation
Phase
Phase 21
Treatment of Severe ACLF
References
Phase 2 equivalent – Randomised controlled clinical trials in patients are usually called Phase 2 trials in the pharmaceuticals industry. Carbalive and Dialive are following a medical device regulatory pathway in Europe, and ‘Phase 2 equivalent’ indicates that have completed randomised controlled clinical trials
NASH – Nonalcoholic steatohepatitis
PSC – Primary Sclerosing Cholangitis





