Pipeline
One critical path.
Three targets.
Three potential treatments.
At Yaqrit we are developing end-to-end solutions for patients with advanced liver disease, built around a deep and new understanding of the role of inflammation and the body’s response to liver injury in the pathogenesis of the syndrome.


Dysbiosis and leaky gut
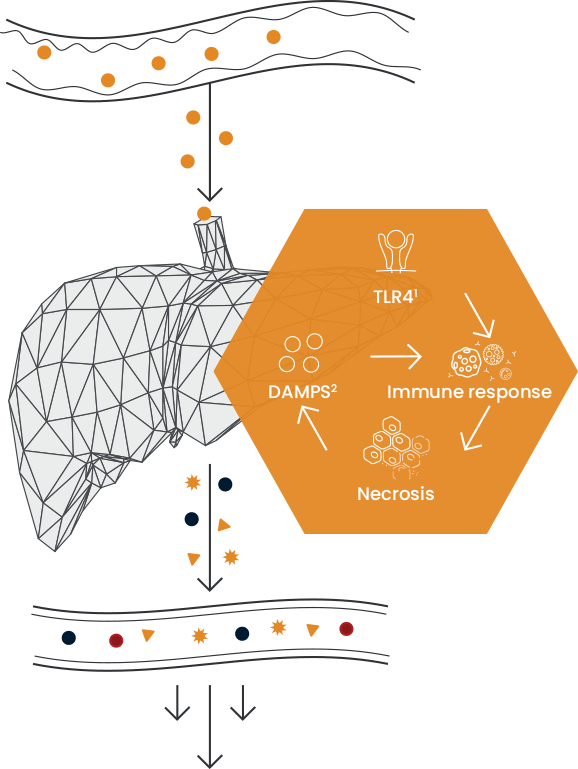
Inflammation leads to a cycle of liver cell death
Loss of liver function leads to further aggravation of liver & other organ(s) failing
Organ Failure
Carbalive
Target 1
Removes endotoxin, reduces bacterial translocation and improves organ function
TLR4 antagonist
Target 2
Reduces sensitivity to endotoxin, modulating inflammation
Dialive
Target 3
Removes endotoxin in the blood and replaces damaged albumin, modulating inflammation
Carbalive
Target 1
Removes endotoxin, reduces bacterial translocation and improves organ function
Organ Failure
Dysbiosis and leaky gut
TLR4 antagonist
Target 2
Reduces sensitivity to endotoxin, modulating inflammation
Organ Failure
Inflammation leads to a cycle of liver cell death
Dialive
Target 3
Removes endotoxin in the blood and replaces damaged albumin, modulating inflammation
Organ Failure
Loss of liver function leads to further aggravation of liver & other organ(s) failing
References
Toll-like receptor 4, a protein in humans that is encoded by the TLR4 gene.
Damage Associated Molecular Patterns.
Macnaughtan J. (2014). Modulation of the gut-liver axis in cirrhosis with activated carbon. Available at: https://discovery.ucl.ac.uk/id/eprint/1547585/1/PhDThesisMacnaughtanFINAL040417.pdf
Carbalive
- Designed to remove harmful bacterial toxins from the gut before they leak to the liver and other organs
- Engineered macroporous carbon beads with a unique physical structure adsorbing both large and small molecules
- Potential oral treatment for previously hospitalised cirrhosis patients who have recovered and are being discharged

TLR4 antagonist
- Targets the TLR4 pathway’s inflammatory cascades and immune responses that drive cell death
- Being developed with Takeda Pharmaceuticals for ACLF patients through a joint venture, Akaza Bioscience
- Yaqrit has plans to develop TLR4 antagonists for other indications such as prevention of ACLF, treatment of hyperammonaemia and urea cycle enzyme disorders
Dialive
- Novel Dual filtration system, similar to a kidney dialysis machine
- Designed to remove bacterial toxins and products of cell death from the blood of patients with advanced liver failure
- Also replaces dysfunctional and harmful albumin

Clinical Development Pipeline
Advancing a clinical stage pipeline designed to address each stage of liver disease from diagnosis of decompensated cirrhosis in a hospital setting, to critically ill patients in intensive care.
Target 1
Removes endotoxin, reduces bacterial translocation and improves organ function
Phase
Phase 21
Decompensated cirrhosis
NASH 2
PSC 3
Target 2
Reduces sensitivity to endotoxin, modulating inflammation
Phase
Phase 2
Treatment of ACLF
Prevention of ACLF
Hyperammonaemia
Urea cycle enzyme disorders
Target 3
Removes endotoxin in the blood and replaces damaged albumin modulating inflammation
Phase
Phase 21
Treatment of Severe ACLF
References
Phase 2 equivalent – Randomised controlled clinical trials in patients are usually called Phase 2 trials in the pharmaceuticals industry. Carbalive and Dialive are following a medical device regulatory pathway in Europe, and ‘Phase 2 equivalent’ indicates that have completed randomised controlled clinical trials
NASH – Nonalcoholic steatohepatitis
PSC – Primary Sclerosing Cholangitis
